Abstract
Failure of response to immunotherapy including checkpoint inhibitors or CAR-T-cell therapy in chronic lymphocytic leukemia (CLL) has been linked to dysfunctional effector T cells. Using single-cell analyses, we performed an in-depth characterization of the T-cell compartment in blood and tissue samples of CLL patients and mouse models to gain insights into the spectrum of phenotypes and transcriptional programs of T cells and the underlying mechanisms of their development.
By mass cytometry (CyTOF) using 35 antibodies, we characterized T cells in blood (n=8), bone marrow (n=3), and lymph nodes (n=21) of CLL patients, as well as reactive lymph nodes of non-tumor patients (n=13). Integrative analyses of all data sets allowed us to identify and quantify 15 clusters of CD4+ and 14 clusters of CD8+ T cells. First, our data showed that T cells in blood and bone marrow are similar, but clearly distinct from lymph node derived cells. Second, we observed an accumulation of several regulatory T-cell subsets, as well as T cells harboring an exhausted and dysfunctional phenotype in the lymph node samples, and a positively correlated abundance of these T-cell subsets. Third, an increased frequency of regulatory and exhausted T cells was detected in CLL in comparison to non-malignant lymph nodes.
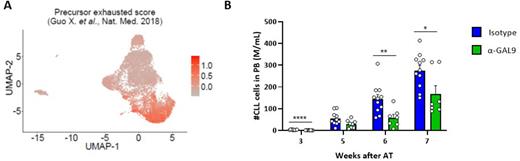
We further performed single-cell RNA-sequencing generating transcriptome and T-cell receptor (TCR) data of T cells from lymph nodes of CLL patients (n=5) and spleen samples of the Eµ-TCL1 mouse model (n=3). These data confirmed the presence of several exhausted T-cell clusters in CLL lymph nodes and spleens of Eµ-TCL1 mice, and allowed for the identification and transcriptional characterization of terminally exhausted T cells and their precursor state (Figure A). By integrating single-cell TCR data, a clonal expansion mainly of the precursor exhausted T cells was observed, suggesting their reactivity for CLL cells.
Finally, we used the single-cell transcriptome data for interactome analyses and identified both known and novel ligand-receptor-interactions between CLL and different T-cell clusters with a presumable function in survival and growth support of CLL cells, but also in suppression of T cells. Among the latter, we focused on galectin-9 which is expressed by CLL cells in patients and mice, and known as ligand for the immunoregulatory receptor TIM-3. We treated mice that had developed CLL after injection of Eµ-TCL1 leukemic cells with galectin-9 blocking antibodies and showed that this treatment slowed down disease development (Figure B).
Altogether, our study provides a detailed characterization of the T-cell compartment in CLL that helps to understand T-cell exhaustion and suggests the TIM-3 ligand galectin-9 as novel target for immunotherapy in CLL.
MZ, EM, JP and MS are co-senior authors of this study.
Figure: A) UMAP of single-cell transcriptome data of T cells from lymph node samples of CLL patients (n=5) displaying a precursor exhaustion score described by Guo et al. in Nat.Med. in 2018. B) Mice that have developed CLL-like disease after adoptive transfer (AT) of Eµ-TCL1 tumor cells were treated with blocking anti-galectin-9 (α-GAL9) or isotype antibodies. CLL cell counts were monitored in blood over time.
Disclosures
Dietrich:Roche: Consultancy; University Hospital Heidelberg: Current Employment; Kite: Consultancy; Gilead: Consultancy. Seiffert:Bayer AG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal